Which Best Compares the Properties of Ionic and Metallic Substances
In a typical ionic solid positively charged ions are surrounded by negatively charged ions and vice-versa. 1 Located in a mobile sea shared by many atoms.
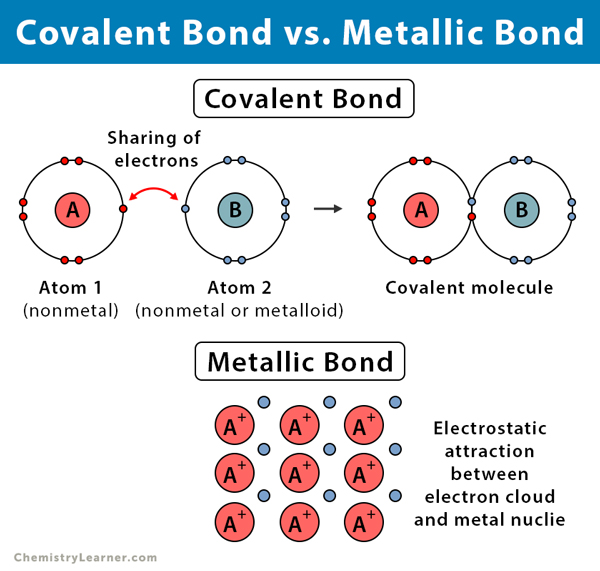
Ionic Covalent And Metallic Bonds Differences And Similarities
3 A metallic substance insulates heat and electricity and.
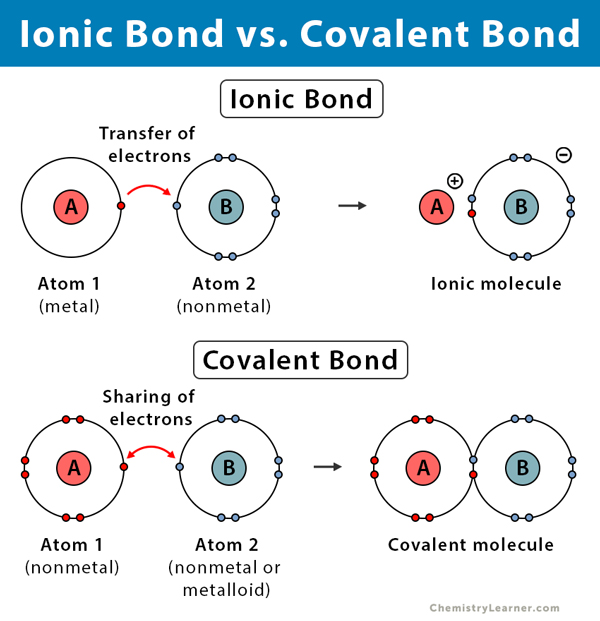
. Group 1 and 2 metals are soft. Bond energy is higher in covalent and ionic bonds than the metallic bonds. Binding energy is higher than the metallic bond.
An ionic bond is always found in compounds composed of both metallic and nonmetallic elements. Ionic compounds consist of ions as individual particles bound in an electronic configuration that is like a stable noble gas. We will discover that the properties of compounds depend upon the type of chemical bond or bonds in the compound.
Solubility in water d. The attraction of metal cationsatoms and delocalized electrons. Conductivity -conduct electricity - do not conduct electricity.
The key difference between ionic and metallic solids is that ionic solids essentially contain cations and anions whereas metallic solids contain metal atoms and free electrons. The bonds of metallic substances are composed of isolated electrons and the bonds of ionic substances are composed of shared electrons. Hence the key difference between ionic bonding and metallic bonding is that the ionic bonding takes place between positive and negative ions whereas the metallic bonding takes place.
Moreover the ionic solids have electrostatic attraction forces between cations and anions while the metallic solids have metallic bonds. 1 Shared unequally by two atoms. Melting and boiling points of the covalent bond are low unlike the metallic bonds and ionic bonds which have higher.
Characteristics of ionic metallic and covalent bonds and compounds. What best compares the properties of ionic and metallic substances. Metallic bonds are malleable and ductile while covalent bonds and ionic bonds non-malleable and non-ductile.
Metals are malleable ductile and have luster. Color - Shiny - opaque. Occurs when 2 atoms share their valence electrons.
Certainly metals are malleable and ductile and are good conductors of heat and electricity whereas ionic solids are frangible and non-conductive and again this is. Properties of Ionic and Covalent Compounds Compare ionic and covalent compounds in the following properties. A metallic substance insulates heat and electricity and solid ionic substances conduct heat and electricity.
Of electrons between the given pairs of atoms thereby. Ionic Solids Ionic solids are solids composed of ionic particles ions. These ions are held together in a regular array by ionic bonding.
Many soluble in non- polar liquids but not in water Ionic Compounds -Formed from a combination of metals and nonmetals. One side has the characteristic. Binding energy is less than covalent and ionic bond.
Recap we have learnt 2 types of bonds exist between compounds Covalent Bonds - Electrons are shared Ionic Bonds - Electrons are Transferred - Balancing char. Electrical conductivity of the compound in liquid form c. 1 Located in a mobile sea shared by many atoms.
Up to 24 cash back Metallic compounds range in hardness. What best compares the properties of ionic and metallic substances. 1 The bonds of metallic substances are composed of delocalized electrons and the bonds of ionic substances are composed of transferred electrons.
When considering the properties ionic solids are hard. Transferred from one atom to another. Group of answer choices.
-Electron transfer from the cation to the anion. The other side has the bondcompound type. IONIC BOND COVALENT BOND METALLIC BOND.
Transition metals are hard. Melting point - high - lower than ionic compounds. The chemical compound formed as a result of transfer.
Group of answer choices A metallic substance has a low melting point and an ionic substance has a low melting point. Form compact arrangement and orderly patterned crystals. Melting and boiling points of the covalent bond are low unlike the metallic bonds and ionic bonds which have higher.
A metallic substance has a low melting point and an ionic substance has a low melting point. 1 Shared equally by two atoms. 2 The bonds of metallic substances are composed of isolated electrons and the bonds of ionic substances are composed of shared electrons.
The bonds of metallic substances are composed of delocalized electrons the bonds of ionic compounds are composed of transferred electrons The bonds of metallic substances are composed of isolated electrons the bonds of ionic substances are composed of shared electrons. Bonding results from the delocalization of valence electrons across the metallic lattice metals tend to have lower melting points. The bonds of metallic substances are composed of delocalized electrons and the bonds of ionic substances are composed of transferred electrons.
Are shared by two atoms. Bond energy is higher in covalent and ionic bonds than the metallic bonds. The ionic bonds result in compounds that are hard and crystalline.
Electrical conductivity of the compound in aqueous solution b. Electropositive element to an atom of a non-metallic. Boiling point - high - lower than ionic compounds.
Because metallic bonding is rather fluid ie. Ionic bonding results from attractive interactions from oppositely charged ions. Metallic bonds are malleable and ductile while covalent bonds and ionic bonds non-malleable and non-ductile.
There is no precise value that distinguishes ionic from covalent bonding but an electronegativity difference of over 17 is likely to be ionic while a difference of less than 17 is likely to be covalent. 6- flammability - not flammable - flammable. In a nonpolar covalent bond electrons are.
Of one or more electrons from the atom of a metallic. Download Therefore the comppund can conduct electricity Covalent compound consists of neutral. 1 Shared unequally by two atoms.
Binding energy is higher than the metallic bond. Occurs during the transfer of electrons. A metallic substance insulates heat and electricity and solid ionic substances conduct heat and electricity.
The chemical compound formed due to mutual sharing. Ionic bonding is a type of chemical bond that occurs between two oppositely charged ions while metallic bonding is the type of chemical bond that occurs in a metal lattice.

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond
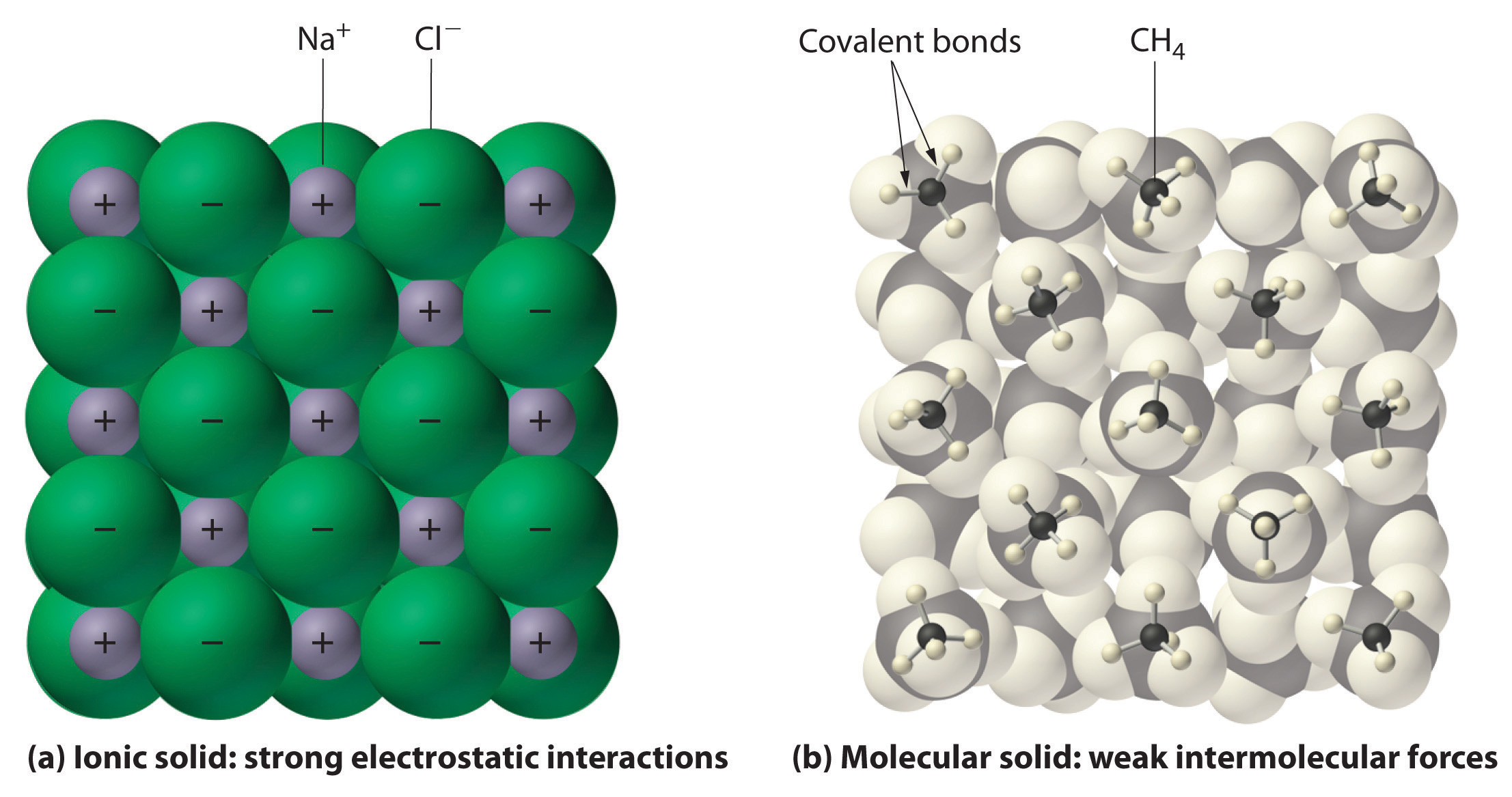
6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts

Ionic Covalent And Metallic Bonds Differences And Similarities

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond
No comments for "Which Best Compares the Properties of Ionic and Metallic Substances"
Post a Comment